
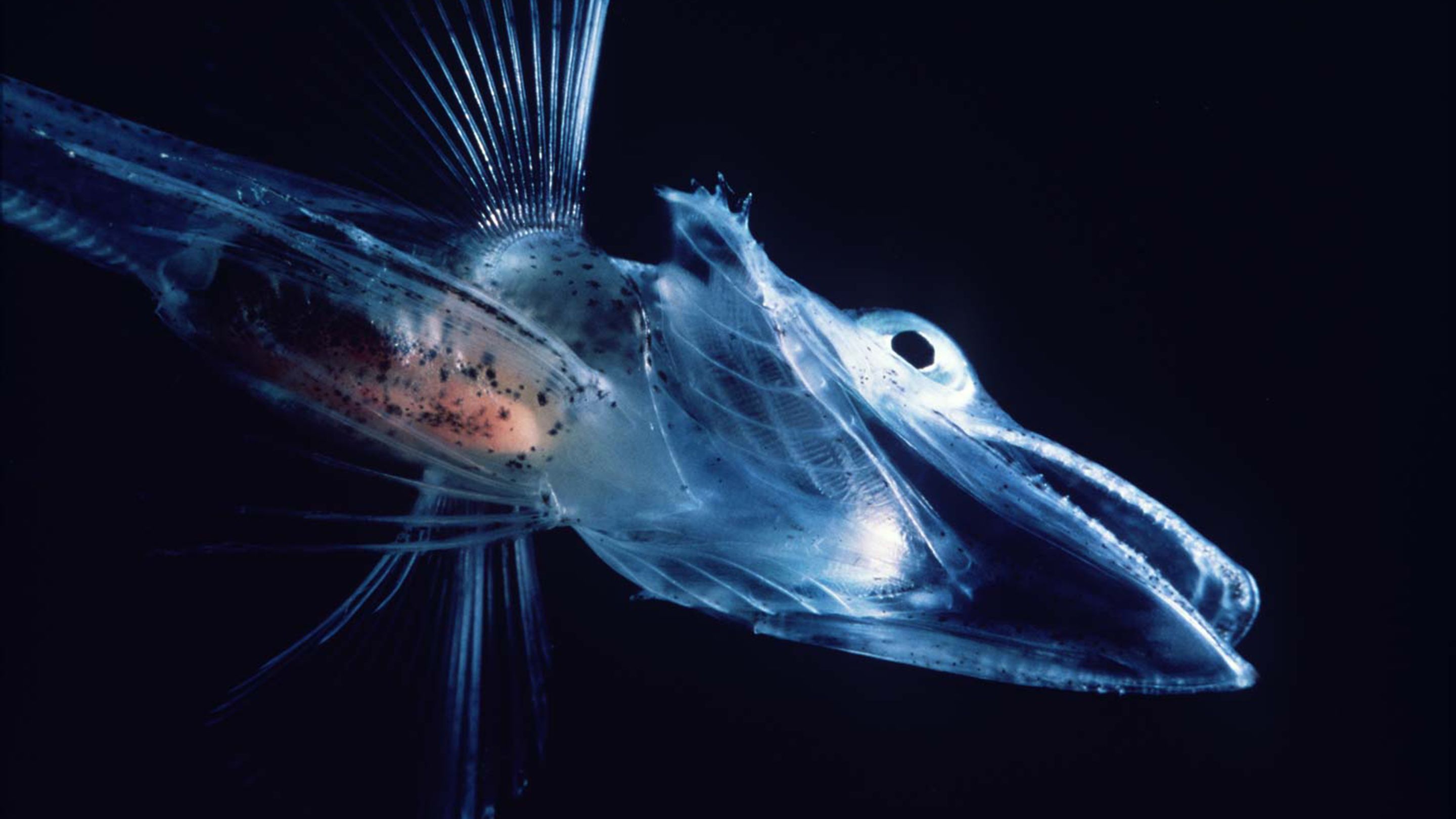
Icefish Study Adds Another Color to the Story of Blood
Living in 10m (32ft) below the surface of the Southern Ocean, where temperatures hover around -2 °C (28.4 F), are fish that seem to be made of the ice they swim beneath. Antarctic icefish are so well-adapted to the frigid waters, they even have an antifreeze glycoprotein in their blood and body fluids to stop ice crystals forming. (source: bbc)
The Antarctic blackfin icefish is the only known vertebrate animal that lacks red blood cells containing hemoglobin. But the use of hemoglobin to transport oxygen through the body is actually a rarity among invertebrates, which rely on a variety of other pigments in their versions of blood.
In February, a genomics study appearing in Nature Ecology & Evolution drew attention to the bizarre Antarctic blackfin icefish, which swim in the brutally cold waters off the coast of the southernmost continent. The icefish of the Channichthyidae family are unusual in several ways — they lack scales and have transparent bones, for example — but what stands out most is their so-called white blood, which is unique among vertebrates. These fish are the only ones known to have neither red blood cells nor hemoglobin pigments for transporting oxygen. Oxygen simply diffuses into their circulating blood plasma from the frigid seawater by way of the fish’s enlarged gills and smooth skin.
By looking at the genome of one icefish species, the researchers were able to peek at the evolutionary adaptations that allowed it to survive. Some were common to red-blooded fish that are also native to Antarctic waters, like the presence of extra genes for making blood proteins that act like antifreeze. Some were more distinctive to the icefish’s lack of red blood cells, such as a boost in the enzymes that protect tissues from the highly reactive free oxygen in its blood.
Odd as the icefish may seem, what makes it peculiar among vertebrates is the norm across the rest of the animal kingdom. Most invertebrates carry genes for hemoglobins, but they generally use other metalloprotein pigments in their versions of blood. Insects, crustaceans and other arthropods use hemocyanin, a bluish copper-based pigment. Mollusks, ranging from clams to squids and octopuses, use hemocyanin, too, but they seem to have invented their version of it independently. Some worms use purplish hemerythrin; others use greenish chlorocruorin; some use a combination of pigments.
It may seem puzzling that so many varieties of blood exist, and more puzzling still that while invertebrates have experimented wildly, vertebrates — aside from the icefish — have stayed universally loyal to the kind with red cells and hemoglobin. The explanation is deeply entrenched in the history of life, going back to the earliest cells.
An Affinity for Oxygen
From the very beginning of life, cells needed to move electrons around between molecules as part of their metabolism, explained Ross Hardison, a professor of biochemistry and molecular biology at Pennsylvania State University. As controls over these redox (oxidation-reduction) reactions, cells deployed ring-shaped molecules called porphyrins. When these porphyrins held a metal atom like iron or copper, they had a ferocious affinity for oxygen. “Once you have an iron in that porphyrin ring, it’s used throughout the biosphere,” Hardison said. He speculated that it “might be one of the earliest molecules that eventually got incorporated into cells.”
Hemoglobin arose out of four interlinked globin proteins, each holding a heme, and it rapidly became ubiquitous. “Hemoglobins predate the origin of animals and even predate the common ancestor of animals and plants,” said Mark Siddall, a curator in the division of invertebrate biology at the American Museum of Natural History.
When respiring animals were only a few cells thick, they could count on diffusion to satisfy their needs for oxygen. But when they grew too bulky for simple diffusion to continue to oxygenate their tissues, hemoglobin was ingeniously ready for the job.
The secret of hemoglobin’s success is collaborative bonding: With every oxygen molecule that the pigment binds, it can bind to the next one more easily, until all four vacancies are filled. This makes hemoglobin extremely efficient at collecting oxygen where it’s abundant (as in the open air and in lungs) and then releasing it again gradually in oxygen-starved tissues.

Vertebrates typically carry genes for several variant globin proteins with finely tuned uses. For example, fetal mammals have a special hemoglobin in their blood with extra affinity for oxygen, which helps them to draw oxygen out of the maternal blood supply in the placenta. Our skeletal muscles make myoglobin, a single globin protein ancestral to hemoglobin, which helps muscle hang on to a reserve of oxygen to use during exercise.
But as good as hemoglobin is, it’s not the ideal molecule for transporting oxygen in all circumstances. Consider hemocyanin, which is so widely used among invertebrates. Hemocyanin is less efficient than hemoglobin at grabbing oxygen because it, like the other hemoglobin alternatives, usually does not bond collaboratively. But the disadvantage of collaborative bonding is that hemoglobin performs worse when oxygen is in short supply. Hemoglobin’s effectiveness also drops with temperature. Consequently, for creatures like octopuses and crabs that live on or near the cold ocean floor, hemocyanin may be a more practical choice.
For insects, it’s different. Their equivalent to blood is hemolymph, a mostly clear fluid that contains small amounts of hemocyanin. But they generally don’t rely on this hemolymph to transport oxygen. Most insects breathe through a network of “tracheal tubes” that pervade their tissues and connect to the air through openings in the exoskeleton. The “open” circulatory system of insects doesn’t have vessels like capillaries to direct the hemolymph; instead, the hemolymph sloshes through the body cavity and helps to distribute dissolved nutrients. The hemocyanin may be in the hemolymph just to help insects store oxygen for later use.
Hemerythrin, the blood pigment found in annelids (segmented worms), leeches and certain other worms, has a deceptive name, because it contains no heme at all. However, like hemoglobin, it is an iron-based pigment descended from a family of ancient proteins that early bacteria used to control redox reactions. Hemerythin has only about one-quarter the oxygen capacity of hemoglobin, though this seems to serve the worms adequately. The pigment also seems to have some immunological functions.
A Toxic Triple Threat
Even if the alternative blood pigments are generally a poor second to hemoglobin at grabbing oxygen, they do have an advantage in terms of simplicity: They usually don’t need something like a red blood cell to hold them. In squids, lobsters and the other blue-blooded animals, for example, hemocyanin is dissolved directly in their plasma. This approach works because hemocyanin, hemerythrin and the other pigments are big, frequently polymerized molecules that keep their oxygen-binding metal atoms tucked away from casual interactions. Conversely, hemoglobin is small and its aggressively reactive heme is easily exposed, which makes it highly toxic — so much so that our livers make a protein, haptoglobin, to scavenge stray hemoglobin from broken blood cells out of our blood.

From a toxicity standpoint, hemoglobin is a triple threat, explained Pampee Young, the chief medical officer of biomedical services for the American Red Cross. Heme has even greater affinity for nitric oxide than oxygen, and the body uses nitric oxide as a signaling molecule to control blood pressure. Excess free hemoglobin will therefore rob the blood of nitric oxide, constrict blood vessels and potentially cause hypertension and reduced blood flow to the organs. Compounding the problem is that hemoglobin, when unprotected in blood plasma, decomposes into its component globin subunits. The naked heme molecules then randomly attack the lipid membranes and other structures in the tissues, damaging them. And as a coup de grâce, the isolated globin proteins can clog the filtration system of the kidneys and shut them down.
Packaging hemoglobin into red blood cells (erythrocytes) helps to contain the toxicity problems. It also makes the distribution of oxygen more efficient by keeping the hemoglobin inside the blood vessels: The molecule is otherwise so small that some of it would leak out into the tissues and fall out of circulation.
The Perils of Justifying Evolution
Human red blood cells are particularly optimized for the job of oxygen distribution. They are compact, flexible and shaped like biconcave disks, which helps them slip through narrow capillaries and gives them a high volume-to-surface area ration, so they can hold a lot of hemoglobin and oxygen. Moreover, human erythrocytes go a step further than those in most species by ejecting their nucleus and other organelles after stockpiling all the proteins they will need for the balance of their lives — what’s left is “basically a bag of hemoglobin,” Young said. The cells pay a penalty for that streamlining, however: Because of their limited ability to repair the wear and tear of pushing through capillaries, circulating human red cells have a lifespan of only about 120 days.
When red cells die, the body converts the hemoglobin down into somewhat less toxic compounds including the green pigment biliverdin. (The green color of a healing bruise is from biliverdin.) Too much biliverdin in a human causes jaundice, but biliverdin is normally present in the blood of certain insects and fish, even though it does not transport oxygen. Last year, the herpetologists Christopher Austin and Zachary Rodriguez of Louisiana State University and Susan Perkins, a parasite researcher in the division of invertebrate zoology at the American Museum of Natural History, reported on their genetic analysis of certain skinks from New Guinea that have so much biliverdin in their blood that its green overpowers the hemoglobin’s red. (“They have something like 50 times the amount of biliverdin that it would take to kill a human being,” Perkins said.) Genetic evidence suggests that this trait evolved four separate times among these lizards, which led researchers to think that the biliverdin might help protect the skinks from malaria or other parasitic infections. Unfortunately for that theory, preliminary evidence suggests that’s not the case, Perkins said, which leaves it mysterious why evolution favors the trait so much in this one small group.
Recommended Videos
 A 100,000 Year Old Electrical Connector Found Embedded In Stone724 views
A 100,000 Year Old Electrical Connector Found Embedded In Stone724 views Amazing! Giant Megabats!108 views
Amazing! Giant Megabats!108 views-
Advertisements
 Fukang Star Stone – the most beautiful meteorite in the world11671 views
Fukang Star Stone – the most beautiful meteorite in the world11671 views The Giant Chinese Dobson Fly (Acanthacorydalis fruhstorferi)92 views
The Giant Chinese Dobson Fly (Acanthacorydalis fruhstorferi)92 views Behold: Lady Gaga's Most Gaga Looks Ever1177 views
Behold: Lady Gaga's Most Gaga Looks Ever1177 views 39 Bird Species That Are Named By People Who Definitely Hate Birds1138 views
39 Bird Species That Are Named By People Who Definitely Hate Birds1138 views Hollyhock (Alcea rosea)55 views
Hollyhock (Alcea rosea)55 views Adorable Portraits of Rescued Baby Sloths221 views
Adorable Portraits of Rescued Baby Sloths221 views




